Roos midfielder opens up on damaging heart complications
https://www.zerohanger.com/roos-midfiel ... ns-118059/
That’s two Aussie rules players that I know of.
Moderators: Elvis, DrVolin, Jeff
Roos midfielder opens up on damaging heart complications
Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs
Received 9 February 2022, Revised 3 April 2022, Accepted 8 April 2022, Available online 15 April 2022.
Handling Editor: Dr. Jose Luis Domingo
Highlights
•
mRNA vaccines promote sustained synthesis of the SARS-CoV-2 spike protein.
•
The spike protein is neurotoxic, and it impairs DNA repair mechanisms.
•
Suppression of type I interferon responses results in impaired innate immunity.
•
The mRNA vaccines potentially cause increased risk to infectious diseases and cancer.
•
Codon optimization results in G-rich mRNA that has unpredictable complex effects.
Abstract
The mRNA SARS-CoV-2 vaccines were brought to market in response to the public health crises of Covid-19. The utilization of mRNA vaccines in the context of infectious disease has no precedent. The many alterations in the vaccine mRNA hide the mRNA from cellular defenses and promote a longer biological half-life and high production of spike protein. However, the immune response to the vaccine is very different from that to a SARS-CoV-2 infection. In this paper, we present evidence that vaccination induces a profound impairment in type I interferon signaling, which has diverse adverse consequences to human health. Immune cells that have taken up the vaccine nanoparticles release into circulation large numbers of exosomes containing spike protein along with critical microRNAs that induce a signaling response in recipient cells at distant sites. We also identify potential profound disturbances in regulatory control of protein synthesis and cancer surveillance. These disturbances potentially have a causal link to neurodegenerative disease, myocarditis, immune thrombocytopenia, Bell's palsy, liver disease, impaired adaptive immunity, impaired DNA damage response and tumorigenesis. We show evidence from the VAERS database supporting our hypothesis. We believe a comprehensive risk/benefit assessment of the mRNA vaccines questions them as positive contributors to public health.
Theresa M Long, MD, MPH, FS
(LTC) •
The opinions expressed are those of my own and do not reflect those of the US Army, the DoD or any entity thereof Aerospace and Occupational Medicine Specialist
15 hours ago
When a commercial airline pilot is forced to get the thing…developed syncope…worked up, cleared to return to flight..has an airbus with 190 souls on board landing in Texas…and has sudden cardiac death immediately after landing…and NOT A SINGLE MAINSTREAM MEDIA WILL COVER THE NEAR CATASTROPHIC AIR DISASTER- wake up something is wrong!
PASSENGERS ON THAT FLIGHT- did you know how close you came to dying??







Belligerent Savant » Sat Apr 16, 2022 8:03 pm wrote:Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs
Received 9 February 2022, Revised 3 April 2022, Accepted 8 April 2022, Available online 15 April 2022.
Handling Editor: Dr. Jose Luis Domingo
Highlights
•
mRNA vaccines promote sustained synthesis of the SARS-CoV-2 spike protein.
•
The spike protein is neurotoxic, and it impairs DNA repair mechanisms.
•
Suppression of type I interferon responses results in impaired innate immunity.
•
The mRNA vaccines potentially cause increased risk to infectious diseases and cancer.
•
Codon optimization results in G-rich mRNA that has unpredictable complex effects.
Abstract
The mRNA SARS-CoV-2 vaccines were brought to market in response to the public health crises of Covid-19. The utilization of mRNA vaccines in the context of infectious disease has no precedent. The many alterations in the vaccine mRNA hide the mRNA from cellular defenses and promote a longer biological half-life and high production of spike protein. However, the immune response to the vaccine is very different from that to a SARS-CoV-2 infection. In this paper, we present evidence that vaccination induces a profound impairment in type I interferon signaling, which has diverse adverse consequences to human health. Immune cells that have taken up the vaccine nanoparticles release into circulation large numbers of exosomes containing spike protein along with critical microRNAs that induce a signaling response in recipient cells at distant sites. We also identify potential profound disturbances in regulatory control of protein synthesis and cancer surveillance. These disturbances potentially have a causal link to neurodegenerative disease, myocarditis, immune thrombocytopenia, Bell's palsy, liver disease, impaired adaptive immunity, impaired DNA damage response and tumorigenesis. We show evidence from the VAERS database supporting our hypothesis. We believe a comprehensive risk/benefit assessment of the mRNA vaccines questions them as positive contributors to public health.
https://www.sciencedirect.com/science/a ... 152200206X
Jeffrey Morris
Director of Division of Biostatistics, Professor
Perelman School of Medicine, University of Pennsylvania
Does a recently published paper by Peter McCullough and colleagues really “establish a mechanistic framework” for mRNA vaccine harm?
This blog post explores that question.
https://www.covid-datascience.com/post/ ... ccine-harm
Comments (sampling):Ian Weinberg
Neurosurgeon and Neuromodulator at Triangles Model Applications (TMA) Academy
It is unfortunate that a not so subtle bias is perceptible in the critique of McCullough's paper. In the face of the shenanigans which occurred in the original vax studies prior to release (as reported in the BMJ), the excess pathologies reported among vaccinees in the US armed forces and the documented excess cardiac pathologies post-vax, the article submitted by McCullough et al becomes extremely relevant. Whether the defenders of the popular vax narrative like it or not, whether challenges to that narrative predispose to vax hesitancy or not, we have validated significant morbidity and mortality associated with the vax which needs to be honestly investigated bias-free. As the medical fraternity, to be committed to 'do no harm', we owe it to our patients and the general public, present and future, to fully investigate the virus, the vax and the entire public health exercise to ensure that we stay true to the science and not allow ourselves to be captured by any alternative narrative and its prevailing bias. I regard McCullough's article as indeed providing a comprehensive framework for the further study of significant adverse consequences of the vax and as such it is an important contribution to the scientific cause.
Re:
Jeffrey Morris [Author]
Director of Division of Biostatistics, Professor
The scientific community is not denying the limitations and risks of vaccines that we have learned about through legitimate scientific process.
Bottom line is they make claims and propose hypothesis but do an exceptionally weak job of providing any direct evidence that they are underlying any effect of Covid mRNA vaccinations.
You may agree with their speculative hypotheses (or like them), but that doesn’t change the fact that this paper from all scientific standards does not support the claims.
Re:
Peter C.
And University of Pennsylvania partnered with Moderna in mRNA vx research, and received funding for same from Pfizer (that's all documented in SEC filings). True, it might be an academic mousehole but the employees are at least savvy enough to understand that biting the hand that feeds them or their employers will never do. We're unlikely to see objective, relevant criticism from University Professors of the official narrative.
Re:
Ian Weinberg
Neurosurgeon and Neuromodulator at Triangles Model Applications (TMA) Academy
Thanks for that Peter. Pretty relevant to the discussion.
Re:
Plamen L. Simeonov
The last sentence of the fifth highlighted by Morris' citation from Seneff's et al. article is more than alarming. An earlier article on interpreting VAERS outcomes by Jessica Rose and Peter MacCullough was banned by ELSEVIER's Cardiology:
https://www.sciencedirect.com/science/a ... via%3Dihub
I know that one of the Elsevier's editors resigned because he could not live with such censure.
....
Re:
Gus Rosania
Professor of Pharmaceutical Sciences at University of Michigan
I thought the burden of proof is on the company that pushed the drug product through the FDA, not in the scientific community. Now what we do not even have placebo controls, how can the “scientific community” establish anything? There simply is no scientific, mechanistic basis for establishing safety. That’s the point. I guess safety is not important any more.
Re:
Jeffrey Morris Author
Director of Division of Biostatistics, Professor
Gus Rosania: it is. That’s why they did randomized phase 3 studies and demonstrated serious adverse events were not any higher in vaccine than placebo arm, and efficacy was demonstrated.
As I said, for all drugs and vaccines post approval safety monitoring is necessary to detect, validate and characterize any minority harm risks that are too rare to every detect in phase 3 studies size.
Re:
Gus Rosania
Professor of Pharmaceutical Sciences at University of Michigan
Jeffrey Morris again those were the ‘vaccine related’ side effects that showed no difference. ‘Vaccine unrelated’ side effects were brushed under the carpet. But, there was no basis for saying what was “related” or “unrelated” to the RNALNP since it is a completely unexplored MOA.
Re:
Victor A.
Principal Partner Research and Analytics Co.
The main message in this paper is that mRNA vaccines weakens the immune system which leads to cascade of events including fuelling of viral mutations, reactivating dormant viruses, reactivation of cancers in remission, inducing cancers due to T cell depletion etc.
I have written some articles in the past that resonates with this paper;
https://victorazodoh.substack.com/p/cov ... -break?s=w
https://victorazodoh.substack.com/p/ope ... ailure?s=w
Re:
Gus Rosania
Professor of Pharmaceutical Sciences at University of Michigan
And I thought the basic idea behind this paper is that we did not have a mechanistic framework to explore mRNA vaccine harm prior to approval without placebo controlled clinical trials, while dismissing such things as “loss of common sense” and “irrational risk taking” as possible mental side effects resulting from altered neuro-immuno-endocrine and inflammatory pathways affecting the CNS.
Re:
Jeffrey Morris
Director of Division of Biostatistics, Professor
No phase 3 trial is ever sufficient to detect and validate all possible vaccine harm, since many of the concerns are very rare, <1/10k or even <1/100k or 1/1m.
That is what post approval safety monitoring, as is being done in this case, is necessary and this is where these rare risks are discovered.
In spite of their claim in the article, it is not the crossover of placebo to vaccine in the trial that necessitates posts approval safety monitoring — it would be required irregardless
Re:
Gus Rosania
Professor of Pharmaceutical Sciences at University of Michigan
Jeffrey Morris the phase3 trials dismissed some side effects as “vaccine unrelated” which is simply a no-no in regulatory science -especially when the potential mechanisms of toxicity were completely unknown since this is a new kind of “vaccine” MOA. Then, throwing away any chance of placebo control is more of a NO-NO so now we are clueless. But? Please correct me if I am wrong.
Re:
Jeffrey Morris
Director of Division of Biostatistics, Professor
Gus Rosania: it is routine in clinical trials to classify events based on likelihood of relatedness, but all adverse events are reported
And it would have been unethical to disallow crossover once the vaccine was available to the public — it is routine to offer crossover at that point — that’s just how it is
Re:
Gus Rosania
Professor of Pharmaceutical Sciences at University of Michigan
Jeffrey Morris: That’s exactly how I see the point of this article. Without placebo control, nobody can really know what side effect is related or unrelated to the LNP construct used in this vaccine.
Re:
Gus Rosania
Professor of Pharmaceutical Sciences at University of Michigan
The burden is on the people at the FDA who approve drug or vaccine products without understanding the potential mechanisms of toxicity. This is not a laboratory experiment with cells or mice. This is a large scale experiment on the human population based on a pharmaceutical product that was approved without a clear understanding of its mechanisms of toxicity nor its addictive potential. Academic scientists who hypothesize a drug product is “safe” are simply approaching drug regulation in the wrong direction. We need to hypothesize a drug product is “toxic” in order to ensure it is safe. One can reject a hypothesis with experiments, but not prove a hypothesis. I guess people have not only forgotten about the importance of drug safety, but they have also forgotten how statistical hypotheses are supposed to serve as guides for scientific experimental design and data analysis. Perhaps “amnesia” should also be included as a potential side effect, in addition to “addiction”? How much do we really know about the effects of the lipids of the LNPs and their downstream neuro-immuno-endocrine effects on dopamine, serotonin and oxytocin telease and reuptake? That’s what I want to know based on some of the comments on this thread.
drstrangelove » 16 Apr 2022 21:13 wrote:Roos midfielder opens up on damaging heart complications
https://www.zerohanger.com/roos-midfiel ... ns-118059/
That’s two Aussie rules players that I know of.
Q: what is ALC-0315 (and SM-102)?
Q: ok, what’s the rub?
Q: hmmm, do we know how toxic? I mean, what kind of toxicity are we talking about?
Q: that sounds…bad. Why isn’t this talked about more by, say, governments, regulators, or medical professionals?
Q: alright, you wanted to bring up something about this, so, any ‘infamous’ one-liners here to sum this up?
Q: well, we all know what happens after that moment, don’t we?
Q: that sounds…appalling. Do you have any evidence to back up your claims?
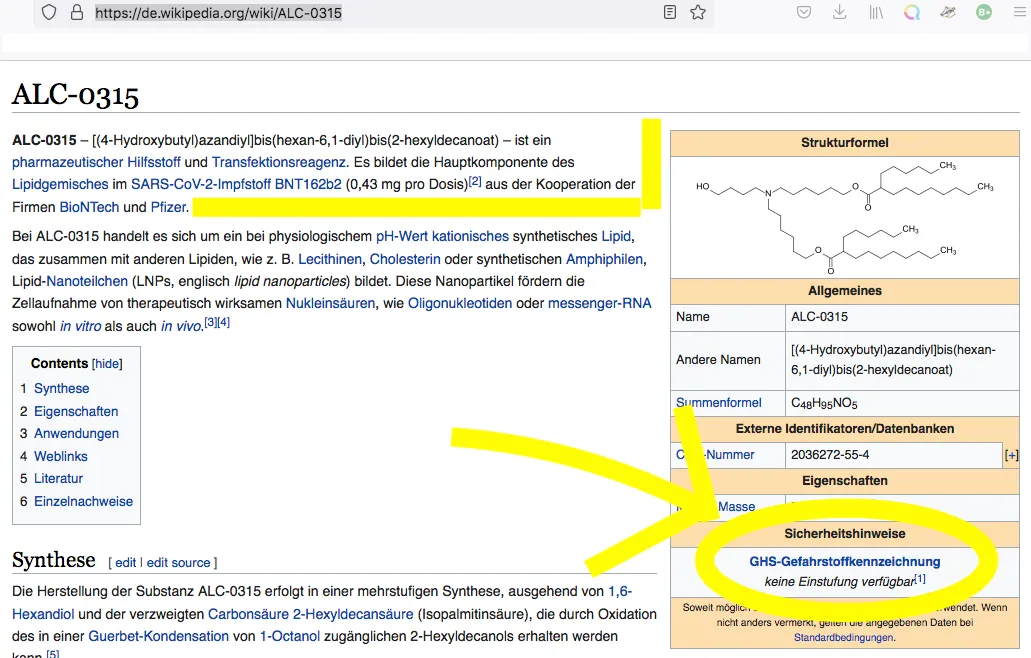
No classification available [keine Einstufung verfügbar]
(1) This substance has either not yet been classified in terms of its hazard or a reliable and citable source on this has not yet been found.
In December 2021 there were objections raised against the use of ALC-0315 and ALC-0159 (and some other solid lipid nanoparticles) in humans by critics of mRNA-COVID-19 vaccines in Germany but the German pharmaceutical trade journal Pharmazeutische Zeitung (de) and the German investigative collective Correctiv refuted this and stated as one important reason that the European Medicines Agency has approved the vaccine and this includes all its ingredients.[9][10]
The lipid nanoparticles in Comirnaty are composed of the components DSPC, ALC-0315 and ALC-0159, and cholesterol, with DSPC and cholesterol being the main components. ALC-0315 and ALC-0159 are two novel lipids, which means they have not been included in any approved drug or vaccine before. The same is true for the two lipids SM-102 and PEG2000-DMG, which are excipients in Moderna's Spikevax®. Since Comirnaty and Spikevax are the first mRNA vaccines to be approved, this is not surprising: these lipids have very specific properties that were not previously required.
The functional lipid ALC-0315 is a novel tertiary amine that is uncharged at physiological pH. During particle preparation under acidic pH conditions, it serves to bind the polyanionic mRNA. After application of the LNP and its cellular uptake into the endosomal cell compartment, it imparts a positive charge to the nanoparticles, which ultimately leads to translocation of the mRNA into the cytosol of the cell. Thus, this lipid is essentially responsible for successful drug application…
The two functional lipids ALC-0315 and ALC-0159 are novel excipients that have not yet been found in approved finished medicinal products.
Pharmacokinetically, the vaccine is characterised by the fact that the highest concentrations are found at the injection site, but a redistribution is observed over time. An accumulation in the liver is typical for nanoparticulate dosage forms and also described for LNP. In pharmacokinetic studies with radiolabelled vaccine, up to 21.5 per cent of the injected dose was detected in the liver and significantly lower amounts in the spleen, adrenal glands and ovaries.
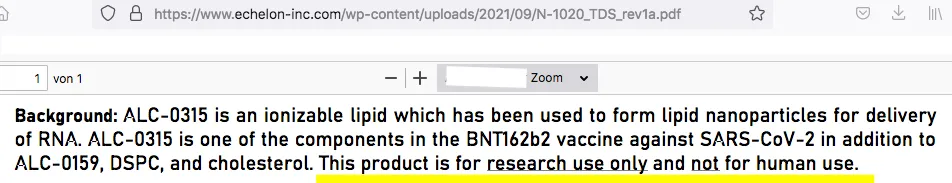
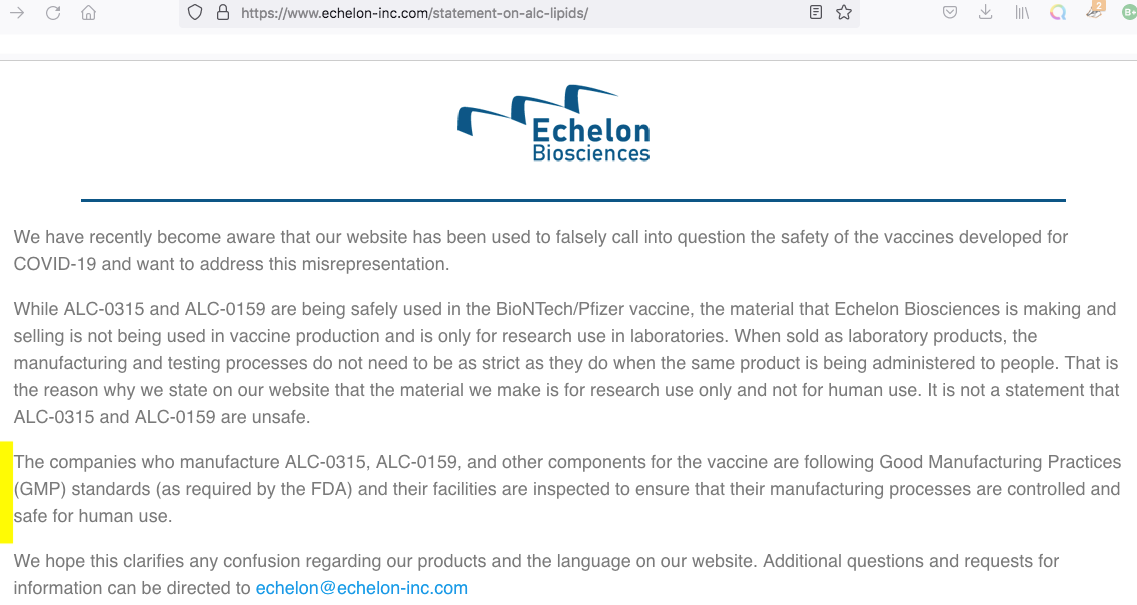
As reported by the website Correctiv, among others, since mid-December [2021] the claim has been spreading on vaccine-critical websites and social networks that the adjuvants ALC-0315 and ALC-0159 are not approved for use in humans. On 22 December, AfD [Alternative für Deutschland] MEP Guido Reil submitted a parliamentary enquiry to the EU Commission to this effect. The lipids ALC-0315 and ALC-0159 used in Comirnaty are produced by the US company Echelon Biosciences and are, according to their information, ‘for research only and not for human use’. The additives are therefore illegal, and the use of the vaccine is ‘illegal, dangerous and unethical’.
Secondly, it is not true that Echelon is the manufacturer of the adjuvants used in Comirnaty—and this is important because the Echelon product information on ALC-0315 and ALC-0159 actually contains the cited clause [i.e., ‘for research use only and not for human use’]. In a statement published on its website in the meantime, however, the company also explains how this is to be understood: the reference is important, it says, because in the case of substances used for research purposes, the requirements for manufacture are less stringent than in the case of intended use in humans.
Is it true that Comirnaty (BioNTech/Pfizer) and Spikevax (Moderna) use ingredients that are not allowed in medicinal products?
No.
The substances ALC-0315 and ALC-0159 in the vaccine Comirnaty (BioNTech/Pfizer) and SM-102 in the vaccine Spikevax (Moderna) are pharmaceutical adjuvants. Pharmaceutical adjuvants can be produced by the drug manufacturer itself or purchased from corresponding companies. Such substances are sometimes offered as laboratory chemicals for a wide variety of applications. The manufacturer usually provides the product information on these laboratory chemicals with a warning that they are not suitable for use in humans. This can lead to the erroneous assumption that they generally cannot be used in humans.
As soon as such substances are used in medicinal products, their suitability for use in humans must be carefully examined and evaluated by the manufacturer and within the framework of the marketing authorisation, e.g. by the Paul Ehrlich Institute. A marketing authorisation application contains corresponding information on quality and production. The above-mentioned testing was also carried out as usual for the approval of mRNA vaccines.
According to the product information supplied by the European Medicines Agency, two of the main components of Pfizer’s Comirnaty vaccine are ALC-0315 and ALC-0159. Echelon, the manufacturer of these nanoparticles, specifies that they are ‘for research only and not for human use’. Administering a vaccine—particularly to children—which contains unauthorised excipients is illegal, dangerous and unethical.
1. How does the Commission justify distributing a product that is harmful to public health and, as such, infringes Article 168(1) of the Treaty on the Functioning of the European Union?
2. How can it explain such a serious oversight—particularly given that the EU founded a European Health Emergency Preparedness and Response Authority (HERA) in September 2021—and how will it avoid similar occurrences in future?
3. What does it intend to do to put an end to the persistent threat that unauthorised vaccine components pose to people in Europe?
The Commission grants a marketing authorisation to a medicinal product based on the objective scientific criteria of quality, safety and efficacy of the medicinal product concerned, following a recommendation from the European Medicines Agency (EMA).
At any time, if new evidence becomes available, EMA assesses it and, if appropriate, recommends the Commission to amend or suspend the authorisation. Comirnaty was granted a conditional marketing authorisation in December 2020, according to this procedure.
A very significant part of the data that any manufacturer of medicines must supply to regulators relates to the quality of the product and its individual ingredients, as well as to showing the necessary manufacturing processes and controls are in place to maintain the quality in every batch of the medicine.
This means that the excipients used in a medicinal product are scrutinised by the regulatory authorities and are part of the authorised composition.
The quality of the ALC-0159 used as an excipient in Comirnaty has been demonstrated to be appropriate for its intended use and is in compliance with the relevant EMA scientific guidelines and standards expected for all medicines.
Details of EMA’s quality and safety assessment of Comirnaty are available in its public assessment report (1).
Other ingredients are: ALC-0315 (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), ALC-0159 (2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide), 1,2- Distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, potassium chloride, potassium dihydrogen phosphate, sodium chloride, disodium phosphate dihydrate, sucrose and water for injections. (pp. 14-15)
All excipients except the functional lipids ALC-0315 and ALC-0159 and the structural lipid DSPC comply with Ph. Eur. The functional lipid excipients ALC-0315 and ALC-0159, are classified as novel excipients…
Two novel excipients are included in the finished product, the cationic lipid ALC-0315 and the PEGylated lipid ALC-0159. Limited information regarding the novel excipients are provided (p. 23)
The proposed specification is considered acceptable based on the available data. However, additional information regarding specifications that should be provided (SO4 [SO is EUrocrat-speak for ‘Specific Objectives’, often used in grant applications and tasks set out by EU institutions]).
Stability data from one supplier indicate that ALC-0315 is stable when stored at the recommended storage conditions. Additionally, the excipient is stable at room temperature suitable for use in further manufacturing steps. Stability data from one supplier is considered representative for lipid from another supplier.
Lipid related impurities have been observed in some recently manufactured finished product batches, correlated with ALC-0315 lipid batches. The quality of ALC-0315 excipient is considered acceptable based on the available data on condition that specific impurities in the finished product will be further evaluated (SO2).
ALC-0315 and ALC-0159 are novel excipients, not previously used in an approved finished product within EU. Additional information is provided separately in Section A.3 of the dossier. (p. 28)
Two novel excipients are included in the LNP. Complete information is not provided for both the cationic lipid ALC-0315 and the PEGylated lipid ALC-0159. In order to assure comprehensive control throughout the lifecycle of the finished product and to ensure batch to batch consistency, further information needs to be submitted regarding the synthetic process and control strategy in line with specific obligations (SO4, SO5).
As regards SO4, the data are requested to be provided regarding the synthetic process and control strategy for the excipient ALC-0315 in order to improve the impurity control strategy, assure comprehensive quality control and batch-to-batch consistency throughout the lifecycle of the finished product.
a) A detailed description of the chemical synthesis of ALC-0315 (e.g., information on reagents and process conditions) should be provided. Due date: January 2021…
e) Specified impurities should be further evaluated and appropriate specification limits for individual impurities should be included when more data are available. Acceptance criteria for specified and un-specified impurities should be added to the specification for ALC-0315 and should also be evaluated during stability studies. Due date: July 2021, Interim report: April 2021…
f) Detailed method validation reports for assay, impurities, and residual solvents for ALC-0315 should be provided. Due date: July 2021
The applicant has determined the pharmacokinetics of the two novel LNP excipients ALC-0315 (aminolipid) and ALC-0159 (PEG-lipid) in plasma and liver as well as their elimination and metabolism in rats. Furthermore, the Applicant has studied the biodistribution of the two novel lipids (in rats) and the biodistribution of a LNP-formulated surrogate luciferase RNA in mice (IV), as well as the biodistribution of a [3H]-Labelled Lipid Nanoparticle-mRNA Formulation in rats (IM).
No traditional pharmacokinetic or biodistribution studies have been performed with the vaccine candidate BNT162b2. (p. 45)
ALC-0315 and ALC-0159 levels in plasma, liver, urine and faeces were analysed by LC-MS/MS at different time-points up to 2-weeks.
ALC-0315 and ALC-0159 were rapidly cleared from plasma during the first 24 hours with an initial t½ of 1.62 and 1.72 h, respectively. 24 hours post-dosing, less than 1% of the maximum plasma concentrations remained. A slower clearance rate was observed after 24 hours with ALC-0315 and ALC-0159 terminal elimination t½ of 139 and 72.7 h, respectively.
Following plasma clearance, the liver appears to be to major organ to which ALC-0315 and ALC-0159 distribute. The applicant has estimated the percent of dose distributed to the liver to be ~60% for ALC-0315 and ~20% for ALC-0159. The observed liver distribution is consistent with the observations from the biodistribution study and the repeat-dose toxicology, both using IM administration.
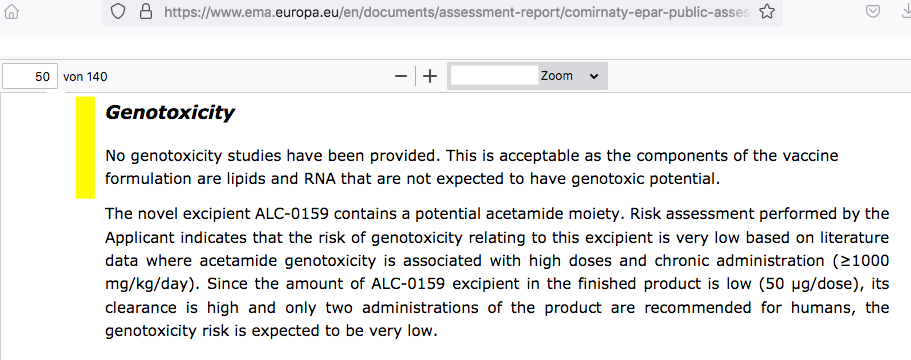
For ALC-0315 (aminolipid), the maximum detected concentration in the liver (294 μg/g liver) was reached 3 hours after IV injection. ALC-0315 was eliminated slowly from the liver and after 2-weeks the concentration of ALC-0315 was still ~25% of the maximum concentration indicating that ALC-0315 would be eliminated from rat liver in approximately 6-weeks. For ALC-0159 (PEG-lipid), the maximum detected concentration in the liver (15.2 μg/g liver) was reached 30 minutes following IV injection. ALC-0159, was eliminated from the liver faster than ALC-0315 and after 2-weeks the concentration of ALC-0159 was only ~0,04% of the maximum detected concentration. The applicant was asked to discuss the long half-life of ALC-0315 and its effect, discussion on the comparison with patisiran, as well as the impact on the boosts and post treatment contraception duration. The applicant considered that there were no non-clinical safety issues based on the repeat dose toxicity studies at doses (on a mg/kg basis) much greater than administered to humans; this was acceptable to the CHMP.
Both patisaran lipids showed an essentially similar PK profile in clinic with a strongly biphasic profile and long terminal half-lives. According to the applicant, it is difficult to further contextualize the pharmacokinetic data and therefore to understand the safety of these molecules, beyond consideration of dose. There is a large dose differential between the human BNT162b2 dose and the dose used in the toxicity studies (300-1000x) which provides an acceptable safety margin.
Moreover, according to the Applicant given the large difference in dose between the toxicity studies and the clinically efficacious dose (300-1000x), it is unlikely that the administration of a booster dose will lead to significant accumulation. Finally, the applicant is of the opinion that these results support no requirements for contraception. The CHMP [which is EUrocrat-speak for ‘Committee for Medicinal Products for Human Use’, of CHMP, is the European Medicines Agency's (EMA) committee responsible for human medicines’] found this position agreeable. (p. 46)
No metabolic studies were performed with the modRNA or the other two lipids of the LNP. Overall, it seems as both ALC-0159 and ALC-0315 are metabolised by hydrolytic metabolism of the amide or ester functionalities, respectively, and this hydrolytic metabolism is observed across the species evaluated.
The metabolism of the novel excipients, ALC-0159 and ALC-0315, were examined in vitro using blood, liver S9 fractions and hepatocytes, all from mouse, rat, monkey and human. The in vivo metabolism was examined in rat plasma, urine, faeces, and liver from a rat pharmacokinetics study where a luciferase-encoding modRNA formulated in an LNP was used.
The toxicological dossier for BNT162b2 is based on a total of three pivotal toxicological experimental studies; two repeat-dose toxicity rat studies and one DART fertility-EFD rat study. The test substance in the repeat-dose toxicity studies is BNT162b2 (100 μg of variant 8 in one study (study 38166) and 30 μg of the clinically relevant variant 9 in the second study (study 20GR142)), which consists of a modified RNA in a lipid nanoparticle (LNP) formulation. The differences between the variants are due to codon optimization. The LNP contains four excipients whereof two are considered novel (ALC-0315 and ALC-0159). (p. 48)
The two general/repeat-dose toxicity studies involved IM exposure of Han Wistar rats to BNT162b2 for a total of 17 days (three weekly administrations) followed by three weeks of recovery. Overall, the study designs only included a single experimental group each with a variant of BNT162b2 (V8 or V9 variant), with no dose-response assessment or specific experimental groups for the LNP alone or its novel excipients. No test substance-linked mortality or clinical signs were observed (except a slight increase [<1C] in body temperature). No ophthalmological and auditory effects were found. The animal model of choice, the rat, has not been assessed in the pharmacological dossier but a limited absorption/ distribution study has been conducted in pharmacokinetics dossier. Immunogenicity was assessed in the toxicology studies.
At 100ug BNT162b2 V8, there were observations of various inflammatory signs at the injection site (e.g. fibrosis, myofiber degeneration, oedema, subcutis inflammation and epidermis hyperplasia). Also, there was inflammation of the perineural tissue of the sciatic nerve and surrounding bone in most rats at d17. The bone marrow demonstrated increased cellularity and the lymph nodes showed plasmacytosis, inflammation and increased cellularity. The spleen demonstrated increased haematopoiesis in half the animals at d17. The liver showed hepatocellular periportal vacuolation at d17 (fully reversed during recovery) which may be related to hepatic clearance of ALC0315. Histopathology assessment of 30ug BNT162b2 V9 generated similar results as 100ug BNT162b2 V8 although not on as extensive level (possibly due to a lesser dose)…
The Applicant explained that peri-portal liver vacuolization was observed in both pivotal studies but are not related to any microscopic evidence of liver/biliary injury in animals (cellular hypertrophy, inflammation) nor any clinical data from Phase 1 study. Vacuoles are considered by the Applicant to be a result of ALC-0315 accumulation in liver and not PEG…
Moreover, increases in neutrophils, monocytes, eosinophils and basophils were observed in study 20GR142. For the Applicant, increases in neutrophils, monocytes, eosinophils and basophils observed in the Study 20GR142 were related to the inflammatory/immune response to BNT162b2 administration.
Similar findings were also identified in Study 38166 in animals administered 100 μg BNT162b2. The applicant stated that the increases in eosinophils and basophils are a minor component of the inflammatory leukogram, which is dominated by increases in neutrophils. The applicant also informed that characterisation of large unstained cells [keep this in mind, cf. below] was not conducted since the identification of these cells does not provide additional information. The CHMP found this agreeable.
Haematology: At 30ug BNT162b2 V9 and 100ug BNT162b2 V8, there was a moderate to strong reduction of reticulocytes (48-74%, not specified for V9) coupled to lowered red cell mass parameters (RBC, HGB, and HCT). There was a moderate to strong increase (>100%) in large unclassified cells [LUC], neutrophils, eosinophils, basophils and fibrinogen that may be related to the inflammatory/immune response.
there was transient reduced body weight gain and food consumption after each dose. No effects on the estrous cycle or fertility index were observed. There was an increase (~2x) of pre-implantation loss (9.77%, compared to control 4.09%) although this was within historical control data range (5.1%-11.5%). Among foetuses (from a total of n=21 dams/litters), there was a very low incidence of gastroschisis, mouth/jaw malformations, right sided aortic arch, and cervical vertebrae abnormalities, although these findings were within historical control data… is noted that there is currently no available data on the placental transfer of BNT162b2.
@gracieinthecity
·
Replying to @AlBowers1 and @dogvoyages
Just a reminder that @dogvoyages HAPPILY GOT THE VACCINE and is now suffering resulting injurious side effects. How exactly does that make her an antivaxxer?
@dogvoyages
·
For those “where’s the proof!?” people. (I redacted this to show somebody else so I might as well post it here). #VaccineSideEffects
@dogvoyages
·
#VaccineSideEffects #MedTwitter #LongCovid
@dogvoyages
·
I’m a “vile attention seeking individual”.
They love you until you get injured- and then they hate you.
@dogvoyages
Bree Dressen gave an amazing interview about the rise of suicides in the vaccine injured, citing that she knows just under 20 people who have killed themselves because of their seemingly never-ending symptoms.
https://greensmoothiegirl.com/your-high ... ne-injury/Ep. 271: Bri Dressen Talks About Suicides of the V-Injured and the Hell Thousands of Injured Have Been Through
Podcast: Play in new window
Over 1.3M injuries have been reported in the U.S., and 24k+ deaths. Today let’s listen with compassion to the story of the life-threatening injury of someone who trusted she was protecting society, by participating in the clinical trial of one of the pharma companies.
LINKS AND RESOURCES:
Watch Video Version Here:
Rumble Video
Bitchute Video
Brighteon Video
**Get this episode’s resources: https://greensmoothiegirl.com/your-high ... ne-injury/
@louietraub
·
29th vaccine injury related doctor appointment: Cardiology rekindled the "anxiety" gaslight, despite being POTS positive and said, "there's nothing more I can do for you." The #VaccineInjured shouldn't only look toward doctors for help, but toward themselves to heal.
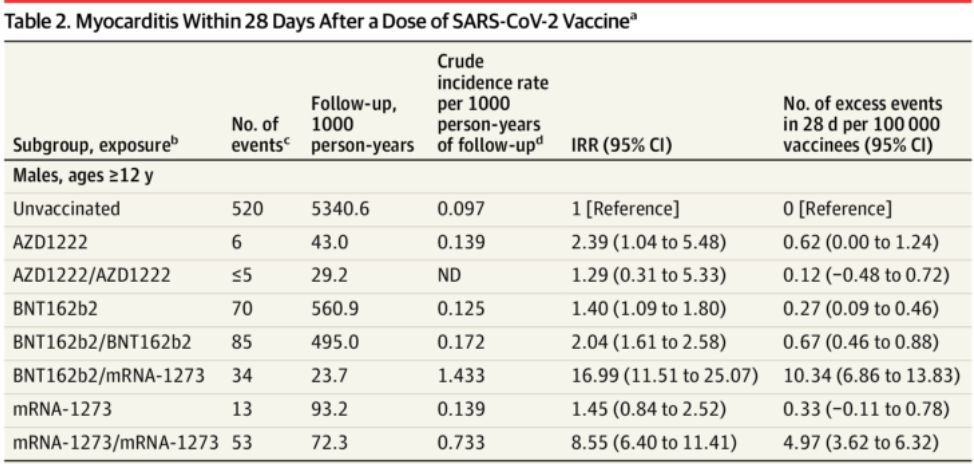
During the 28-day risk period, we observed 105 myocarditis cases following administration of the first dose of BNT162b2 [Pfizer] and 115 myocarditis cases following the second dose. We also observed 15 myocarditis cases following administration of the first dose of mRNA-1273 [Moderna] and 60 myocarditis cases following the second dose.
Among 23,122,522 Nordic residents (81% vaccinated by study end; 50.2% female), 1,077 incident myocarditis events and 1,149 incident pericarditis events were identified. Within the 28-day period, for males and females 12 years or older combined who received a homologous schedule [two vaccine doses of the same type], the second dose was associated with higher risk of myocarditis, with adjusted IRRs [incidence rate ratios] of 1.75 (95% CI [confidence interval], 1.43-2.14) for BNT162b2 [Pfizer] and 6.57 (95% CI, 4.64-9.28) for mRNA-1273 [Moderna]. Among males 16 to 24 years of age, adjusted IRRs were 5.31 (95% CI, 3.68-7.68) for a second dose of BNT162b2 and 13.83 (95% CI, 8.08-23.68) for a second dose of mRNA-1273, and numbers of excess events were 5.55 (95% CI, 3.70-7.39) events per 100,000 vaccinees after the second dose of BNT162b2 and 18.39 (9.05-27.72) events per 100,000 vaccinees after the second dose of mRNA-1273. Estimates for pericarditis were similar.
stickdog99 » Mon Apr 25, 2022 4:50 am wrote:So you have a link for that feed?
C. M. Boling
@dogvoyages
Dog mom, nomad, vanlifer, vaccine injury survivor. Belonging everywhere and nowhere She/Her
Nomad
Joined December 2021
91 Following
5,843 Followers
@dogvoyages
·
21h
Every time I post proof, they move the goalposts. I have documented every single day of this reaction on social media. I will post as much “proof” as I can- because our lives depend on people believing us. Please trust the people you love when it happens to them.
Belligerent Savant » 26 Apr 2022 16:24 wrote:Apologies for my earlier reaction, stickdog. I didn't see your handle and assumed it was an attempt to call out the tweet as "disinfo" or "fake news", etc., rather than earnest inquiry. My error in assuming, regardless of user name behind the query.
I'd like to revise my prior posting, but can no longer do so. Probably better: a reminder for me to be a better human, even in small ways.
Users browsing this forum: No registered users and 7 guests